Self-poisoning for self-preservation – the function of Streptomyces nano-syringes
A novel role for molecular nano-syringes found in the antibiotic-producing bacteria Streptomyces has been revealed.
These structures are known as Contractile Injection Systems (CIS) and are used by many bacteria as effective weapons to inject toxins into rivals.
In Gram-negative bacteria, this can occur due to the nature of their thin cell envelopes, meaning that CIS can penetrate the bacteria and deliver their toxin. But how and why these molecular syringes exist and function in the more complex, Gram-positive, Streptomyces with their thick cell envelope was unknown.
Researchers from the John Innes Centre and ETH Zürich set out to understand this and used a combination of microbiology and high-resolution cryo-electron microscopy to make a series of unexpected findings.
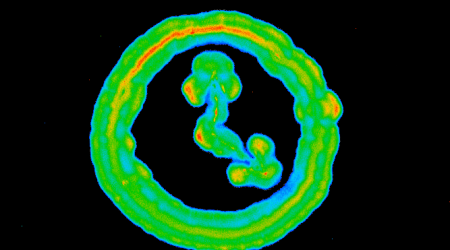
Their first discovery was to show that the CIS produced in Streptomyces coelicolor, float within the cells, and are not embedded in the surrounding cell membrane.
Previous studies on CIS found them either embedded in the cell membrane, ready to fire outwards upon touching other bacteria, or released outwards to target and inject toxins into competing microbes.
The second discovery was to uncover a previously undescribed role in bacteria. The collaborative team revealed that the free-floating CIS in Streptomyces facilitate cell death in response to external stress.
The team identified several toxic molecules that are loaded into the CIS, which assist in killing off cells.
In experiments where the Streptomyces were chemically stressed, the team observed that cells without CIS died less than those with functional CIS.
This led the team to hypothesize that CIS play a role in lysing older or damaged cells, a mechanism which may seem like self-sabotage, but in fact provides two major benefits.
By initiating cell death, the organism can release cellular material to be recycled, feeding other cells within the colony. Secondly, killing off cells or segments of the colony could limit the scale of damage within the complex network of Streptomyces filaments, protecting the wider colony.
Co-author of the study, Dr Joseph Sallmen explains: “Streptomyces are intriguing bacteria, and the more we study them, the more they surprise us. Our research on the Streptomyces CIS showed us that these bacteria have evolved some unique strategies for self-preservation.”
The research concludes that this self-poisoning strategy could provide Streptomyces with a fitness advantage that ensures survival and timely progression through the developmental life cycle which is tightly linked to the production of antibiotics.
The next stage of the research will be to understand the exact mechanism of action of the CIS within Streptomyces: how these free-floating CIS are recruited to the membrane of stressed or old cells to kill them off.
Dr Susan Schlimpert, group leader, at the John Innes Centre said: “Bacteria have a range of solutions to different environmental and cellular challenges. In this work, we describe a previously well-described competition tool that has at least two seemingly unique responses in the filamentous Streptomyces bacteria.
“CIS impact the timely progression through the developmental lifecycle, and they are important in ensuring the bacterium survive stressful growth conditions. This illustrates the importance of increasing our understanding of the diverse roles that related cellular components can play in different bacteria.”
Cytoplasmic contractile injection systems mediate cell death in Streptomyces has been published in Nature Microbiology https://www.nature.com/articles/s41564-023-01341-x