Meet the Molecules: Mirror molecules in plants and fungi
Plants and fungi are very often tangled together in nature.
They are either friends (forming mutually beneficial partnerships) or foes (plant defence vs pathogenic fungi).
Plants use photosynthesis to synthesise and produce organic substances whereas fungi are decomposers living on organic substances
Plants are believed to be very distant to fungi which are deemed more closely related to animals.
These two distantly related organisms have co-evolved with each other for hundreds of millions of years and established very distinctive metabolisms. Yet interestingly, plants and fungi in some cases, can ‘work out’ their own ways to make structurally very similar, mirror, or even identical molecules.
One notable example has been the plant hormone gibberellins, which promote plant growth. Another very recent addition is the discovery of new-to-nature fungal-type ‘sesterterpenes’ from Brassicas by researchers led by Professor Anne Osbourn at the John Innes Centre (1).
Sesterterpenes are a rare subgroup of ‘terpenes’, which are the largest class of small organic molecules produced by plants.
Terpenes have been used extensively as aroma and flavours, cosmetics, agrochemicals and drugs. Sesterterpenes were often found in filamentous fungi and marine sponges but rarely isolated from plants before.
As such, the discovery of plants’ latent capacity to produce sesterterpenes could open new avenues to access an untapped chemical reservoir.
A recent study from the Anne Osbourn Laboratory identified a number of plant genes for the biosynthesis of sesterterpenes, by mining 55 plant genomes.
Functional analysis of seven of these sesterterpene biosynthetic genes revealed the production of eight new-to-nature sesterterpene molecules.
Intriguingly, these newly identified sesterterpenes are structurally reminiscent to those previously isolated from fungi in that they share common molecular skeletons.
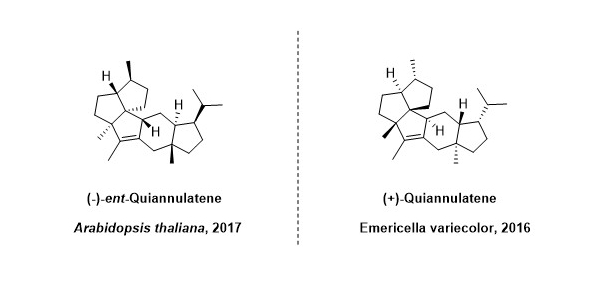
One notable compound, named ent-quiannulatene, is produced by the root of model plant species Arabidopsis thaliana. The slender, threadlike fungi Emericella variecolor could produce a molecule (named quainnulatene) with an identical planar (2D) structure to the plant one (ent-quiannulatene). However, their 3D structures are not identical but are instead mirror images of each other, just like our left and right hands (2).
Furthermore, plants and fungi have evolved different mechanisms to make such mirror molecules, that, being fungi, use only one single gene to do the job of two plant genes.
The reasons why plants produce ‘fungal-like’ molecules are yet to be uncovered. However, sesterterpenes previously isolated from fungi and marine sponges have a wide spectrum of bioactivities ranging from anti-inflammatory, antimicrobial and phytotoxic to antitumor activities.
As a result, sesterterpenes are increasingly regarded as a potential new source of anticancer drugs.
With more plant genomic sequences being made available, the hidden chemical diversity of sesterterpenes in plants will be further unveiled.
This fresh source of chemical entities harbours largely unexplored bioactivities and can be fed into the drug-discovery pipeline. It is therefore likely that we could find some sesterterpene-derived medicines or household and cosmetic products in the market in the near future.
References
- A. C. Huang et al., Unearthing a sesterterpene biosynthetic repertoire in the Brassicaceae through genome mining reveals convergent evolution. Proceedings of the National Academy of Sciences 114, E6005-E6014 (2017).
- M. Okada et al., Genome-based discovery of an unprecedented cyclization mode in fungal sesterterpenoid biosynthesis. J. Am. Chem. Soc. 138, 10011-10018 (2016).
