Solving the puzzle of how connections between plant cells open and close in response to disease-causing microbes.
A plant’s ability to sense and respond to microbial attacks is crucial to protecting it from disease. At a global level, understanding plant immunity will help us to protect the crops that feed the world.
Through this greater understanding of plant immunity, we can improve disease resistance inside plants, with the aim of reducing chemical inputs currently used to control plant disease.
However, each individual plant cell has the ability to sense and respond to attacks. A plant’s immune system involves receptors on both the surface and inside its cells that sense molecules from microbe threats.
These receptors trigger a set of molecular and chemical defences inside the cells. As a result, research on the plant immune system has tended to have a single-cell perspective.
But the Faulkner lab at the John Innes Centre is filling the gaps in our understanding of how cells communicate and coordinate their responses when they sense danger.
“Every cell can perceive a pathogen and trigger a response, but that cell isn’t isolated from the rest of the plant,” explains Dr Christine Faulkner.
Plants are multi cellular, so a single-cell isn’t the full story, and we need to put it in the context of all the other cells.
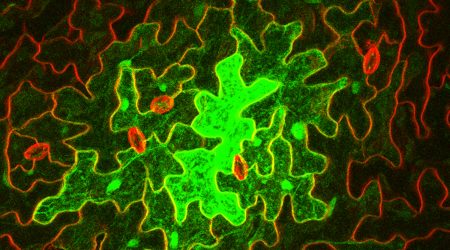
“The gap that the Faulkner group focuses on is plasmodesmata – small channels that directly connect the liquid content of cells to that of their neighbours.
Dr Faulkner was first intrigued by the puzzle of plasmodesmata as an undergraduate student.
“I stumbled upon plasmodesmata because one of my professors, who later became my PhD supervisor, presented the big, unanswered questions of plant-cell biology – the things we don’t know. Plasmodesmata are one of those areas we don’t understand, and my interest has been maintained because they’re still one of the most enigmatic aspects of a plant.
“I’m most excited about figuring out what’s going through plasmodesmata because they are dynamic. They open and close, so they must be controlling the transport of something, but we still don’t really understand what that is”.
The enigma surrounding plasmodesmata is partly due to the challenges of studying them.
remarks Dr Faulkner.
The connecting channels are essential to plants, which means it’s difficult to perform the usual genetic experiments of blocking their production to figure out their function.
As Dr Faulkner explains, “No plant without plasmodesmata has ever been identified. Because the plasmodesmata are so essential, it’s challenging to disturb them to investigate what they do”.
Alongside this challenge, there are difficulties in studying a single boundary between cells in plant tissue that is three-dimensional.
Plasmodesmata are too small to image with a light microscope, but electron microscopes, which provide images at a closer scale, require dead plant tissue, which prevents capturing the dynamics of the plasmodesmata opening and closing
So how does the group overcome these hurdles?
“A lot of what we do is indirect. In our lab we look particularly at what is going through plasmodesmata to try to figure out whether they’re closed or open”, explains Dr Faulkner.
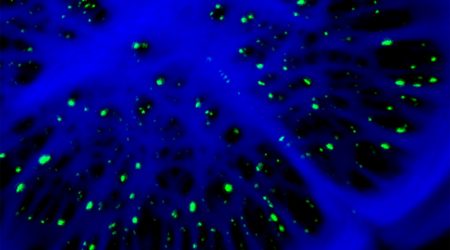
“The team often genetically transform an individual cell to produce a fluorescent protein and examine how far it spreads between cells”.
These experiments are supported by state-of-the-art technology platforms at the John Innes Centre, particularly the bioimaging platform.
Here, the team use techniques such as FRET-FILM imaging, which allows them to detect protein-protein interactions and requires specialised lasers, photon detectors and technical expertise.
“The John Innes Centre has one of the best suites of microscopes in the country and that enables us to collaborate to use advanced and expensive technologies. For us, the Bioimaging platform is essential”.
Through these experiments the Faulkner lab is uncovering the role plasmodesmata have in sensing and responding to microbial attacks.
Early work by Dr Faulkner revealed that during an attack plants try to shut down the plasmodesmata, while the microbes try to keep them open so they can spread throughout the plant.
Building on this, the group has since found that chitin, a fungal material, triggers different responses in the membrane that lines the plasmodesmal tunnels, compared to those it triggers in the membrane around the rest of the cell.
Delving further, the group’s research has shown that guard-like receptors that sit in the plasmodesmata are different to those in the rest of the membrane, but both types of receptor use the same enzymes to signal downstream.
Together, this suggests that the plasmodesmata open and close independently of the rest of the immune response.
“To me, it seems like a trade-off. The cell will always perceive the signal and trigger an immune response, but whether or not the plasmodesmata close is maybe dependent on whether the cells need to connect for resources”, says Dr Faulkner.
This concept is perhaps more relatable than ever amid the Covid-19 pandemic. “I think of it as an emergency isolation – it’s like a lockdown”, explains Dr Faulkner.
“We can isolate ourselves away, but if we don’t have a food supply, that’s no good. We’re going to have to open back up and go to the shop to get food supplies. So possibly, it’s the same thing for the cell. If it has lots of supplies the cell can close the doors and get the benefit from that.
“We think there’s something going on because there’s got to be some benefit to having two completely independent signalling pathways.
“So, I think that’s one of the hey areas we’d like to focus on as we move forwards, because that pertains to the whole question of multi cellularity”.
Communication within science, too, is important for the future of the group’s research and the John Innes Centre as a whole, amid proposed plans for a new plant and microbial science hub in Norwich.
Dr Faulkner is an adjunct scientist at our sister institute The Sainsbury Laboratory (TSL), which shares our joint vision.
“TSL works on lots of different areas of plant immune responses, so that complementarity, and anything that facilitates it, amplifies what we can do”, says Dr Faulkner.
Wounds and waves
Plants sense damage to tissue whether by microbes, organisms or other factors.
This is the focus of Annalisa Bellandi’s research, who has recently completed her PhD in the Faulkner group, “My PhD focused on cell-to-cell transmission of calcium waves in plants. When plants perceive stimuli, they produce signals that move cell to cell and organ to organ (e.g. leaf to leaf) allowing information to travel and trigger responses to the stimulus across the whole plant”.
Through damaging plants, either in major ways such as cutting off leaves or in smaller ways using microneedles, and imaging and analysing calcium waves, Annalisa has developed a fuller picture of how plants sense wounding.
“It’s exciting to study this on different scales – starting at the cellular level with studying subcellular localisation of molecular components involved in the transmission of the waves all the way to the wider level, with the vascular and organ-to-organ transmission.”