Circadian clock clue to infection mechanism in the malaria parasite
A discovery first made in plants has inspired an important insight into the mechanism by which the malaria parasite can infect humans and animals.
The research, a collaboration between Tokyo Institute of Technology, Nagasaki University, and the John Innes Centre, offers a future target for malaria drug discovery.
Professor Antony Dodd, a group leader, at the John Innes Centre and one of the authors of the study says, “It is amazing that a process we identified in plants has led to the discovery of an equivalent mechanism in a globally important pathogen.”
Previously Professor Dodd’s group at the John Innes Centre had shown that a protein encoded by the cell nucleus, called a sigma factor, is necessary for the regulation of some genes in the chloroplast, the site of photosynthesis in plants.
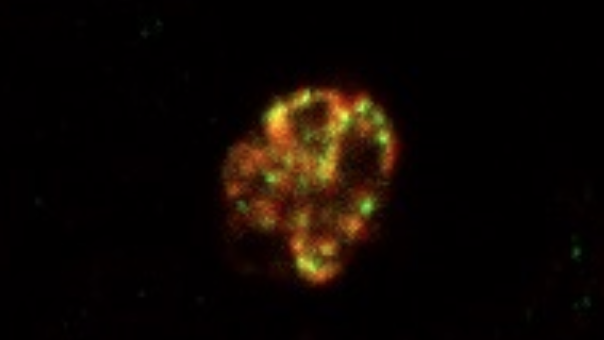
This led them to wonder whether comparable sigma factors exist in the malaria-causing parasite Plasmodium falciparum. These parasites have an interesting evolutionary history which means their cells contain a chloroplast-like compartment called the apicoplast which contains its own genome.
Using a combination of genetic analysis and immunofluorescence microscopy to study P. falciparum in the lab, the research team identified a Plasmodium sigma factor (ApSigma) that binds to the apicoplast genome and regulates gene expression. Data suggested that the sigma factor is essential for parasite survival.
Experiments showed that expression of the apicoplast genes increased in the presence of melatonin, the mammalian circadian signaling hormone present in host blood that is produced in response to darkness and regulates sleep.
This suggests links between the host circadian rhythm and the parasite gene regulation.
By identifying a mechanism by which the parasite lifecycle and host body clock are synchronised, this study opens new opportunities for anti-malarial treatments.
Professor Dodd says: “The new protein and mechanism identified could present a new target for the development of drugs for the treatment and or prevention of malaria, in both humans and farm animals.
Malaria is one of the biggest public health risks, with around 240 million people from across the globe contracting it every year. Potentially life-threatening, it is transmitted by the bite of a female Anopheles mosquito infected with Plasmodium falciparum.
This parasite enters the human body through the mosquito bite and causes symptoms like fever, cold, fatigue, and headache, which are highly periodic.
The periodicity of symptoms can be linked to the synchronization of the parasite’s life cycle with the circadian rhythm — the 24-hour internal biological clock—of the infected person or the host.
Professor Kan Tanaka from the Tokyo Institute of Technology highlights the future implications of the present research. “Malaria kills hundreds of thousands of people across the world, every year. This study identifies a regulatory system that might be a future target for malaria treatment.
Professor Dodd adds: “This research demonstrates the value of international and interdisciplinary collaboration, and the power of plant sciences and microbiology to drive unusual and novel discoveries that could be of considerable global benefit.”
Co-ordination of the apicoplast transcription in a malaria parasite by internal and host cues appears in PNAS (Proceedings of the National Academy of Sciences).



